
You are:
The DEHP-free, BPA-free INFINEED lt;Gravity lt; 3-way infusion set has been designed to minimize its environmental impact. Two directions have been taken: the first by reducing the mass of plastics used by around 10%, without any change in functionality, and the second by using exclusively endocrine-disruptor-free raw materials (eliminating components containing DEHP or BPA).
The polycarbonate-free 3-way stopcock has been tested at pressures well above those recommended for gravity perfuion; laboratory tests have shown a pressure resistance of around 10 bar
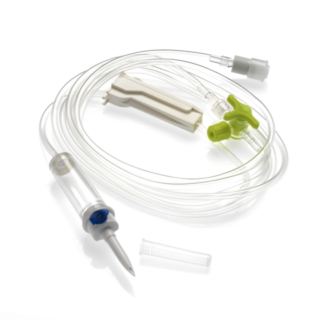
Perfuser with :
- Two-channel perforator
- Air intake (0.45µm filter): Supplied closed for immediate use on bag.
- Chamber: Flexible and translucent, the chamber is calibrated to 20 drops = 1ml ± 0.1ml. 15µm particle filter.
- Caster: Precise and reliable, linear ABS flow regulator provides good rigidity.
- BPA-free 3-way tap: Lipid-resistant, with wide key and flow-indicator arrows, non-removable and integral with the extension. Only the main line can be disconnected from the valve.
- Tubing: PVC (DINCH ) designed for optimum comfort and adjustment regularity and to avoid kinks. Diameter: 3 x 4.1mm.
- End cap: Luer Lock mobile
- End cap with 0.8 µm purge filter (Hydrophobic membrane) for automatic purging of the infusion line.
Length: 150 cm + 30 cm
Residual volume: 15.8 ml
Made in China.
Contact with 0.5% or 2% Chlorhexidine in 70° Isopropyl alcohol, without evaporation time, while the luer lock is connected, and therefore under physical stress, presents a significant risk of luer lock cracking, and should be avoided wherever possible.
In line with SF2H (2019) recommendations, disinfecting connectors (when necessary) with 70° ethyl alcohol is always preferable, as it is just as effective at disinfecting inert materials, and with much less risk of altering plastics.
Indications
This device enables gravity administration of injectable parenteral preparations: drugs, solutions, parenteral nutrition.
According to good perfusion practice, the infusion line can remain in place for up to 96 hours (CODIMS, OMEDIT CENTRE-VAL-DE-LOIRE, Socle de connaissance de la perfusion - SFAR, EUROPHARMAT, [...]).
In addition, the SF2H reminds us not to change the main infusion line before 4 days, without exceeding 7 days.
This device has been tested for pressure, traction and lipid resistance for up to 7 days.
The length of time the infusion line should be maintained should be discussed and validated in each facility, in order to establish an internal protocol.
Gravity infuser, not to be used for pressurized infusion, especially contrast media injection. Not to be used for transfusions, or for drugs requiring a 0.2µm filter.
Dispose of in accordance with local regulations.
Used materials
PVC DINCH (DEHP <0.1%(m/m)): Chamber, air intake, Tubing - Polypropylene: tap cap - Polyethylene: tap key, HDPE: Protective cap for the perforator - Tritan® BPA free: Stopcock body - Acrylonitrile Butadiene Styrene: Perforator, regulator body, wheel, mobile Luer Lock - Polyamide: anti-particle filter at the bottom of the chamber - Polyethylene/PTFE: purge filter
Labeling
on each unit are written the reference, the lot/serial number, the sterilization method and the expiry date if applicable
medical device
sterile
conformity
economic operator
Transport conditions
Protect from light and humidity and store at a temperature between 5°C and 20°C.
| Conditioning | Parts quantities | Dimensions in cm | Weight in kg | Ean 13 of conditioning |
| UNIT | 1 | 21 x 10 x 1 | 0.029 | 3661809029356 |
| BAG | 25 | 48 x 28 x 5 | 0.800 | 3661809129353 |
| CASE | 250 | 59 x 39 x 27 | 9.100 | 3661809329357 |