
You are:
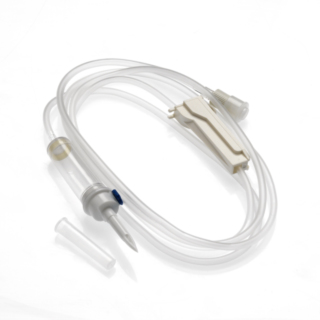
The INFINEED sterile, DEHP free, BPA free 1-way infusion device has been designed to minimise its environmental impact, using only raw materials that are free from endocrine disruptors (elimination of components, including DEHP or BPA). Residual volume: 230 ml Made in China.
Infuser with:
- Two-channel perforator with grip wings.
- Air intake: Delivered closed for immediate use on a 0.2µ filter bag.
- Chamber: Flexible and transparent, Calibrated at 20 drops = 1ml ± 0.1ml, 15µ particle filter.
- Flow regulator: Accurate and reliable, linear flow regulator.
- Tubing: PVC (DINCH or TOTM) designed for comfort, optimal flow adjustment consistency and to avoid kinking.
- End fitting: Mobile Luer Lock.
- End stopper: Equipped with a purge filter with anti-bacterial membrane of 0.8µm
Length: 300 cm
Contact with 0.5% or 2% Chlorhexidine in 70° Isopropyl alcohol, without
evaporation time, and while the luer lock is connected, therefore under
physical stress, presents a significant risk of cracking of the luer
lock, and should be avoided as much as possible.
In
accordance with the recommendations of the SF2H (2019), disinfecting the
connectors (when necessary) with 70° ethyl alcohol is always
preferable, as it is just as effective in disinfecting inert materials
and there is much less risk of altering plastic materials.
Indications
This device enables the parenteral administration by gravity of injectable preparations: parenteral and photosensitive drugs, solutions and nutrition when long tubing is required.
According to good perfusion practice, the infusion line can remain in place for up to 96 hours (CODIMS, OMEDIT CENTRE-VAL-DE-LOIRE, Socle de connaissance de la perfusion - SFAR, EUROPHARMAT, [...]).
In addition, the SF2H reminds us not to change the main infusion line before 4 days, without exceeding 7 days.
This device has been tested for pressure, traction and lipid resistance for up to 7 days.
The length of time the infusion line should be maintained should be discussed and validated in each facility, in order to establish an internal protocol.
Dispose according to regulation in member state.
Used materials
PVC (DEHP < 01.% (m/m)): Chambre, prise d'air, tubulure - DINCH : plastifiant - Polyéthylène : Filtre de purge, capuchon protecteur perforateur, Luer Lock mobile - Acrylonitrile Butadiène Styrène : Perforateur, régulateur de débit linéaire - Polyamide : Filtre à particule
Labeling
on each unit are written the reference, the lot/serial number, the sterilization method and the expiry date if applicable
medical device
sterile
conformity
economic operator
Transport conditions
Protected from light, humidity and heat
| Conditioning | Parts quantities | Dimensions in cm | Weight in kg | Ean 13 of conditioning |
| UNIT | 1 | 0.040 | 3661809032738 | |
| BOX | 50 | 24 x 39 x 29 | 3.740 | 3661809032745 |
| CASE | 200 | 42 x 50 x 40 | 9.620 | 3661809032752 |